
Steam Purity
As a general rule of thumb, a steam purity study can help assure efficient plant operation, but how is this conducted and what does steam purity refer to?
Steam purity refers to the condition of the steam in regards to the purity of the steam chemistry as ppb of TDS, sodium, cation conductivity, or silica in the steam. This is a measurement of the amount of solid, liquid, or vaporous contamination present in steam. There are several factors that can contribute to the degradation of purity including carryover, entrained water, priming, foaming, etc. High-purity steam will correlate to little contamination present. It is important that a steam purity measurement be periodically conducted as impurities can foul superheater tubes which can result in their ultimate rupture, cause turbine deposits, and potentially interrupt plant processes which require the use of steam. The terms “steam purity” and “steam quality” are not interchangeable. Steam quality is a measure of the moisture content within the steam and is typically expressed as the weight of dry steam in a mixture of steam and water.
Conducting a steam purity study involves the proper collection of steam samples, the accurate analysis of those samples and the establishment of the proper operating conditions to achieve meaningful results. However, accurate sampling can be difficult to achieve due to fine water droplets being uniformly dispersed within the steam line. For this reason, Pitot tube sampling devices are recommended for accurate testing in supply tubes and superheater inlet tubes. Years of research conducted on proper sampling nozzle design and location have resulted in nozzle designs specified by ASTM and ASME.
Several methods of measuring steam purity have been established, of which include TDS, specific conductance, sodium tracer, and silica content. An adequate amount of baseline data is necessary to be able to properly evaluate a potential carryover situation which may require collection and analysis of steam at various boiler loads.
TDS & Conductivity
Measuring specific conductance or TDS in the steam is the most simplistic method for determining steam quality as a conductivity value is proportional to the concentration of ions present in the sample. The influx of TDS, possibly as a result of boiler carryover, can easily be tested and acknowledged. Measurement of such increase provides a rapid and reasonably accurate method for determining steam purity. One disadvantage of conductivity is that some dissolved gasses commonly present in steam, such as ammonia and CO2, ionize in water. This interference can lead to an increase of conductivity. Degassing sample equipment can be utilized to avoid such interferences.
Sodium
Online equipment, such as sodium ion analyzers, have proven to be reliable and provide continuous monitoring. In typical operation, a regulated amount of an agent such as ammonium hydroxide is added to a regulated amount of condensed steam sample to raise pH and eliminate the possibility of hydrogen ion interference. A reservoir stores the conditioned sample and feeds it at a constant flow rate to the tip of the sodium ion electrode and then to a reference electrode. The measured electrode signal is compared to the reference electrode potential and translated into sodium ion concentration, which is displayed on a meter and supplied to a recording device. Sodium analyzers are popular due to their simplicity and ease to keep operational with repeatable results within 0.1 ppb. Sodium in steam can come from the boiler chemical treatment program such as sodium phosphate or from the alkalinity contributed by sodium hydroxide.
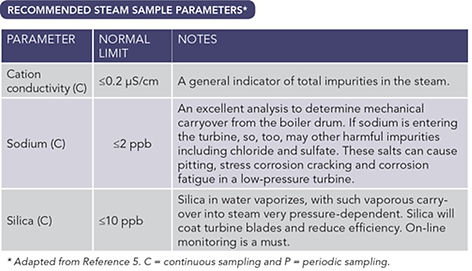
Monitoring sodium can identify chemical and mechanical boiler errors. Elevated sodium content in steam can cause deposition and can indicate the presence of other impurities such as chlorides and silica. Testing for anions such as chloride and sulfates can provide another means of steam purity monitoring. Measuring the degassed cation conductivity will give a measurement of the total anion concentration. Chlorine and silica electrodes are available, although online analyzers are expensive and difficult to keep operational.
There are several potential causes of poor steam purity. Operating variables such as maintaining an elevated steam load above the boiler design could lead to carryover. It is important that there remains steam space in the boiler for separation to occur. Foaming and priming may lead boiler water to rock and sequentially reduce steam purity. Of course, poor water treatment can cause elevated chemical contamination, excessive alkalinity, or sodium content. Thankfully all of these influential parameters can be adjusted, but without proper steam monitoring, contaminated steam can cause severe damage to turbines, leading to lengthy unplanned outages, expensive repairs, and loss of revenue.
List of Resources:
[1] Dunham, M. (2004). Steam Purity [Slides]. Columbia Water Technology. http://www.boiler-wrba.org/2016Presentations/13-BoilerSilicaCarry-Over.pdf
[2] Water Handbook - Steam Purity | SUEZ. (2000). Suezwatertechnologies. https://www.suezwatertechnologies.com/handbook/chapter-16-steam-purity
[3] Monitoring Steam Purity in Power Plants Part 1: Using Conductivity. (2016). Emerson. https://www.emerson.com/documents/automation/application-note-monitoring-steam-purity-in-power-plants-part-1-rosemount-en-73098.pdf
[4] Committee, R. A. (1995). A Practical Guide to Avoiding Steam Purity Problems in Industrial Plants (40th ed.) [E-book]. ASME Press. https://doi.org/10.1115/1.I00383_ch6
