
Softened Water Makeup in Cooling Towers
It is commonly accepted throughout the water treatment industry that softened water should not be the sole provider of makeup water in cooling towers, but is this justified?
The evaporation/heat transfer that occurs within a cooling tower system converts relatively soluble calcium and alkalinity from the raw water into insoluble calcium carbonate. Provided flow rates are high, these scale-producing minerals ideally are carried in suspension until removed by bleed-off or filtration. However, calcium carbonate can settle in areas of low flow and begin to accumulate on heat transfer surfaces. These resulting calcium carbonate deposits form hard crystalline structures on the walls of heat transfer locations, drastically reducing efficiency and performance.
Although utilizing softened water may limit scale production, the adverse effect is true with corrosion. Eliminating calcium (Ca²⁺) and magnesium (Mg²⁺) will result in an ionic imbalance resulting in an aggressive water. A visual representation of corrosion tendencies can be shown using the Ryznar Stability Index (RSI).
To compensate for this corrosive tendency, cooling towers must operate at high cycles of concentration. More concentration cycles will reduce the bleed rate but will promote the rapid accumulation of environmental dirt and debris in the system which can harbor microbiological growth. A strong dispersant program is needed under these conditions along with frequent manual tower cleanings. There are however cooling systems that can operate with softened water as water sources vary drastically throughout the nation.
The alternative route is to apply a chemical treatment that will counteract the formation of hard scale or deposits and modify them in a way that disrupts the crystalline structures they can form. There is a plethora of crystal modifiers/sludge conditioners capable of distorting scale growth on the market today.
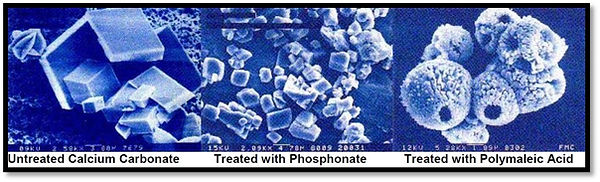
Figure 1: Scanning Electron Micrographs Comparing Crystal Structures

Figure 2: Chemical Structures of Some Common Scale Modifiers
As it does not take very much material to distort a crystal structure, these species are effective at sub-stoichiometric concentrations, i.e., far below the concentrations that would be expected on a molecular level. The scanning electron micrographs in (Figure 1) show some structures that were obtained under lab conditions and the effectiveness of any product is dependent upon the system where it is applied.
Under normal operating procedures I would agree that, exceptions aside, it is more beneficial to use hard water for cooling water operations. Where the makeup water is exceedingly hard and there is a shortage of water, partial softening can be a useful tool (mixing valves piped to allow for some hardness to remain present). As always, consult a professional water treatment before making these decisions.

