
Chlorides in Water Treatment
As a general rule of thumb, testing Chlorides in the water treatment industry can be an important parameter, but why?
Generally speaking, the presence of chlorides in boilers, cooling towers, and closed loops has few performance benefits or disadvantages. At extreme concentrations, chlorides may contribute to corrosion and scale formation if conditions are suitable, which is quite rare. Chlorides are widely distributed in nature as sodium salts (NaCl), potassium (KCl), and calcium (CaCl2) and typically are leached from various bedrocks into soil and water as they naturally weather. So, if chlorides are found universally in our water systems and do not necessarily contribute to corrosion or scale formation in our systems, why bother even testing them?
The ASME guideline set for chlorides in most boiler settings is not to exceed 300 ppm (mg/l) as steel components can then become sensitive to stress corrosion cracking. Chlorides are quite soluble, and for that reason do not typically contribute to scale formation. Although this should be a threshold that needs to be taken into consideration, it is rare that chlorides will be the limiting parameter. Nonetheless, if left unmonitored can lead to internal issues. As it relates to water treatment, chlorides are the best parameter for determining cycles of concentration in water treatment applications as they are the most stable and soluble component when compared against other water impurities. Chlorides are not destroyed by steam and elevated temperatures and are not typically altered by chemical additions. From accurately testing for chlorides throughout a boiler system; percent makeup, blowdown, concentration cycles, and several other valuable calculations can be performed.
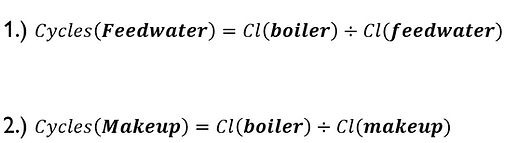
From the above equations, percent makeup water can then be calculated:
By simply conducting chloride tests in several samples, many useful calculations can be performed, allowing water treaters to accurately predict the amount of chemical required along with potential savings that can be implemented with any program adjustments. These calculations also apply to cooling systems where chlorine/bromine is not being utilized. In addition, the presence of chlorides in returning condensate can indicate carryover or intrusions of other liquids as they should not be present in steam.
Chlorides are typically sampled by method of titration with a silver nitrate solution and chromate as an indicator. As the silver nitrate solution is added, a precipitate of silver chloride forms. The endpoint occurs when all of the chlorides are precipitated and potassium chromate forms, creating a reddish-brown silver chromate. It is important to note that orthophosphate and polyphosphate may interfere if present greater than 250 ppm. As the reaction is pH-dependent, anything that precipitates at an elevated pH (such as hydroxides) may interfere. This is why sulfuric acid should be added before the titration to avoid the production of silver carbonate.

I’ll admit, continuously sampling for chlorides can get quite repetitive and redundant at times where the make-up water quality is consistent. Chlorides, in my opinion, can become an underrated parameter if not at least periodically tested. Chloride testing is a must when initiating a water treatment program for the valuable calculations that can be performed. Taking the time to perform the mentioned calculations not only allows us to better understand each system, but to also proactively prevent any issues from occurring.
List of Resources:
[1] Wilson, M. (2019, January 28). How do I control the chloride in my boiler water? – Restaurantnorman.com. Restaurant Norman. Retrieved November 15, 2021, from https://www.restaurantnorman.com/how-do-i-control-the-chloride-in-my-boiler-water/
[2] Wu, J. (2019, March 11). chloride in boiler water – Industrial Boiler Supplier. Boiler Supplier LTD. Retrieved November 15, 2021, from http://www.boilersupplier.ltd/chloride-in-boiler-water.html
[3] Boiler Water Tests. (n.d.). Viking Water Technology, Inc. Retrieved November 15, 2021, from http://vikingwater.com/boiler-water-tests/
[4] Boiler water chemistry - Properties of feed water explored. (2009, July 16). Bright Hub Engineering. Retrieved November 15, 2021, from https://www.brighthubengineering.com/marine-engines-machinery/42291-properties-and-effects-of-feed-water-treatment/
[5] THE BASICS OF COOLING TOWER WATER TREATMENT. (n.d.). Flozone. Retrieved November 15, 2021, from https://www.flozone.com/wp-content/uploads/2014/07/basics_cooling_tower
